How mRNA Could Defeat Latent Viruses and Train Immunity Against Cancer
Moderna and BioNTech deploy AI-driven mRNA to target CMV, EBV, HSV, and personalized neoantigen cancer vaccines. These trials could redefine immunotherapy.
Welcome back to Biotech Blueprint!
You can listen to this episode on YouTube, Spotify or Apple Podcasts.
Check out our newly updated website biotechblueprintconsulting.com.
In Part 2 of our mRNA miniseries by Biotech Blueprint × Biotech Capital Compass, we dug into mRNA’s next frontier: latent viruses like CMV and EBV, and personalized cancer vaccines that retrain the immune system to recognize mutated cells as threats. It’s a pivot from seasonal prevention to long-term immune engineering.
Why latency is so hard to erase
“Latent” means hidden or dormant, because viruses like CMV, EBV and HSV slip into long lived cells and stay there for life, reactivating when the immune system gets distracted. Antibodies can’t reach them because there’s nothing floating around to attack. That job falls to T cells, which must find and destroy infected cells.
The problem is that most traditional vaccines don’t train T cells well, but mRNA vaccines might. They can encode multiple viral proteins and deliver them in a way that prompts both antibody and cellular (B and T cell) immunity. That’s what makes it a strong candidate to finally go after viruses we’ve somewhat accepted as permanent houseguests.
CMV as the proving ground
Cytomegalovirus is one of the most common infections you’ve never thought about, and the leading infectious cause of birth defects in most of the world. Moderna’s candidate, mRNA-1647, delivers six mRNAs encoding gB and the pentamer complex, aiming to block viral entry across multiple cell types. The pivotal phase 3 CMVictory trial is ongoing. A December 2024 Data and Safety Monitoring Board interim found the early efficacy threshold was not met but recommended the study continue as planned. Final efficacy readout is anticipated in late 2025/early 2026.
The CMVictory trial is more about CMV. It’s a stress test for the entire mRNA platform. If it works, it proves mRNA can handle complex, multi-antigen vaccines that elicit broad T cell immunity and durable protection. It would also open the door to EBV and HSV, both biologically trickier, but now technologically feasible.
EBV, HSV, and the overlooked upside
Epstein-Barr virus infects up to 90% of adults. For some, it manifests as mononucleosis, followed by a lifetime of dormancy. For others, it raises risks for certain cancers and is suggested to be a trigger for multiple sclerosis. Moderna’s two EBV vaccines (one to prevent infection, another to suppress reactivation) could rewrite disease risk decades later.
HSV, meanwhile, remains stubbornly common (HSV-1 affects an estimated 64% of worldwide population, while HSV-2 about 14%) and under discussed. Even modest reductions in reactivation and transmission would deliver meaningful quality of life and public health gains.
These programs were long ignored not because the targets lacked value, but because the technology couldn’t handle the biology.
From pathogens to tumors
Cancer flips the equation. We’re not blocking infection anymore, but retraining surveillance. Personalized neoantigen vaccines like Moderna’s mRNA-4157 (V940) sequence a patient’s tumor, select unique mutation-derived targets (neoantigens), and encode them into an mRNA shot. With Keytruda removing PD-1 brakes, vaccine-primed T cells expand and stay active against tumor neoantigens.
Phase 2b melanoma data showed a 49% reduction in recurrence or death versus Keytruda alone, which is striking. Phase 3s are expanding to lung and bladder cancers. It’s personalized medicine in the most literal sense: one batch per patient.
At this year’s ESMO Congress (Oct 17-21) in Berlin, Moderna unveiled early data from its investigational cancer antigen therapy mRNA-4359, a dual-target mRNA that encodes epitopes of PD-L1 and IDO1 to retrain the immune system against tumors that have escaped prior checkpoint inhibition. In a small phase 1/2 study of heavily pretreated melanoma patients, the mRNA-4359 + Keytruda combo achieved a 24% overall response rate and 60% disease-control rate, rising to 67% in PD-L1-positive tumors, alongside evidence of antigen-specific T cell expansion and new T cell receptor clones. This hints at genuine on-target reprogramming rather than nonspecific immune rescue. Safety was manageable with no new immune-related adverse events, and median duration of response was not yet reached. Now moving into phase 2 testing across melanoma and NSCLC, mRNA-4359 reinforces Moderna’s broader thesis: that its mRNA platform can do more than express viral antigens, it can re-code immune recognition itself.
Why Moderna keeps showing up in this story
Moderna’s edge is infrastructure. AI-assisted antigen selection, automated manufacturing, and a rapid design→build→test loop that moves from sequence to shot in weeks. That engine is why the company keeps surfacing in any serious discussion of mRNA beyond COVID.
Yes, it’s also been one of the most shorted large cap healthcare stocks, with short interest hovering around 18-19% of float. Bears have clear reasons: post-COVID revenue compression, cost cuts and 10% workforce reductions, and an event-driven pipeline where pivotal readouts (CMV, oncology) dictate timelines and cash burn.
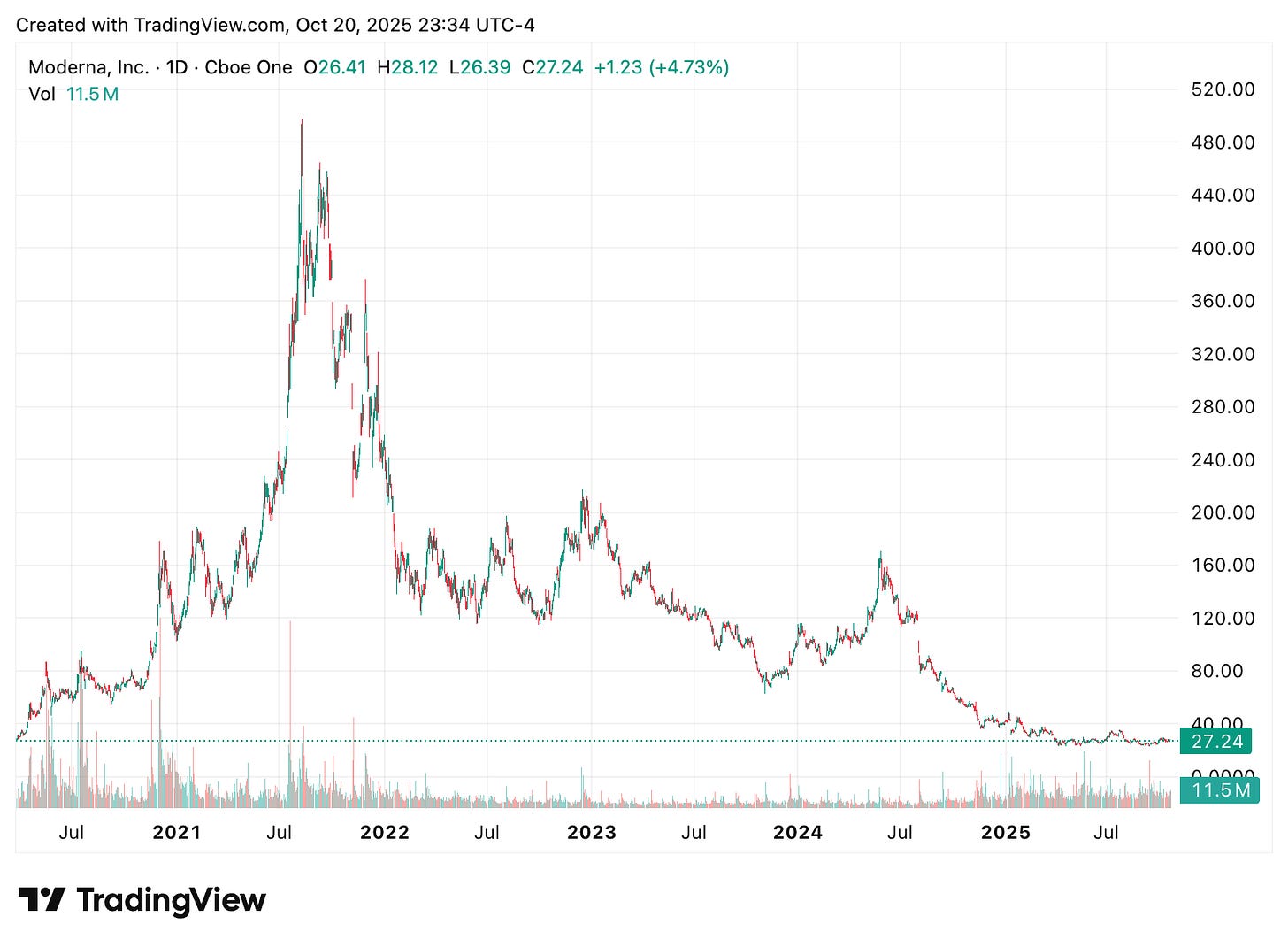
The long-term case is still straightforward. Multiple shots on goal with public-health and commercial upside. The CMV phase 3 (mRNA-1647) is a platform stress test with meaningful burden-of-disease upside if it works. The personalized cancer neoantigen vaccine has shown durable, 3-year benefit in melanoma and is already in phase 3 across bigger tumor settings. If those programs read out well, the narrative shifts from “COVID company” to “programmable immunity platform.”
What success would mean
Clinically: Vaccines evolve into long term T cell trainers, not just seasonal antibody boosters.
Public health: Fewer congenital CMV cases. Over time, potential reductions in EBV-linked cancers and MS risk.
Operationally: Clear playbooks for multi-antigen and personalized products, from sequencing logistics to FDA endpoints.
Financially: Platform economics compound. Data from one program improves the next. The mRNA thesis moves from model to medicine.
Connecting the dots
mRNA started with respiratory viruses. The next test is latency and oncology: durable T cell control for CMV/EBV/HSV and individualized neoantigen vaccines for solid tumors. If those trials read out well, the case for mRNA as a general-purpose immunology platform gets much stronger.
Thanks for reading & listening to Biotech Blueprint!





Likewise, thanks for engaging. I enjoyed our conversation
I really liked the article, but can we stop inserting "AI" in places where it's absolutely unnecessary and adds no information?